

The Latest
-

DHS says travelers with no REAL ID can fly for now, but with likely extra steps
Secretary of Homeland Security Kristi Noem said travelers who have not obtained a REAL ID by the deadline can fly after additional identity checks
-

Bruins GM Don Sweeney gives head coaching search update
Bruins general manager Don Sweeney shared a new coaching search update after the results of the 2025 NHL Draft Lottery.
-

MLB Power Rankings roundup: Red Sox slipping amid inconsistent start
Here’s how the Red Sox are viewed nationally amid an up-and-down start to their 2025 campaign.
-
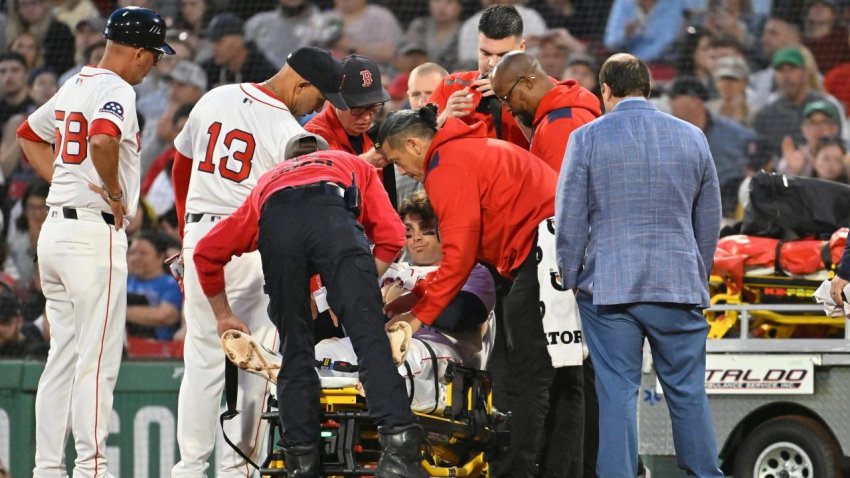
Ranking Red Sox' best Triston Casas replacement options at first base
Who should take over for Triston Casas as the Red Sox’ everyday first baseman? We ranked Boston’s options from best to worst.
-

Friends to enemies? Tom Thibodeau's basketball journey was shaped by time in Boston
He might have orchestrated a Game 1 win for the New York Knicks against the Boston Celtics, but coach Tom Thibodeau’s basketball journey is deeply embedded in Massachusetts.
-

Celtics' issue in Game 1 loss to Knicks was quality, not quantity
The Celtics’ poor 3-point shooting wasn’t the only reason they lost Game 1 to the Knicks. Chris Forsberg breaks down Boston’s vexing defeat.
-

Patriots reveal jersey numbers for 2025 rookie class
The Patriots’ 2025 rookie class has new jersey numbers for the upcoming season.
-

Last location of Boston-area pizza chain closing
What was once a small group of pizza spots will soon officially disappear from the local landscape.
-

Dobbs: You can tell Patriots QB Drake Maye ‘loves football'
Joshua Dobbs shares his early impressions of Patriots QB Drake Maye and how they’ve built their relationship so far.
-

OG Anunoby might be biggest X-factor in Celtics-Knicks series
The Knicks’ best chance to upset the Celtics revolves around OG Anunoby’s play at both ends of the court.
-

The two sides of Celtics' historically bad shooting in Game 1 vs. Knicks
What should we make of the Celtics missing an NBA-playoff-record 45 3-pointers in Game 1 vs. the Knicks? The stats tell two different stories.









