Federal health regulators are warning consumers to stop using certain brands of eyedrops because of potential fungal and bacterial contamination.
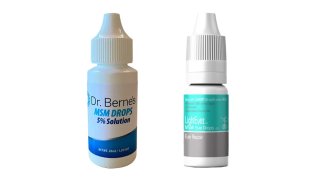
The Food and Drug Administration advised people to not buy Dr. Berne's MSM Drops 5% Solution and LightEyez MSM Eye Drops-Eye Repair drops. The agency said using the products could pose a serious health risk, including vision- and life-threatening infections.
The eyedrops are sold online, but are considered illegal because they contain methylsulfonylmethane, or MSM, an ingredient that is not approved for use in the U.S. and illegally marketed.
The FDA says Dr. Berne agreed on Aug. 21 to voluntary recall the Dr. Berne’s MSM Drops 5% Solution, while LightEyez Limited has not responded to the agency or taken steps to protect consumers.
The FDA urges consumers who are in possession of the drops to dispose of them by following these instructions.
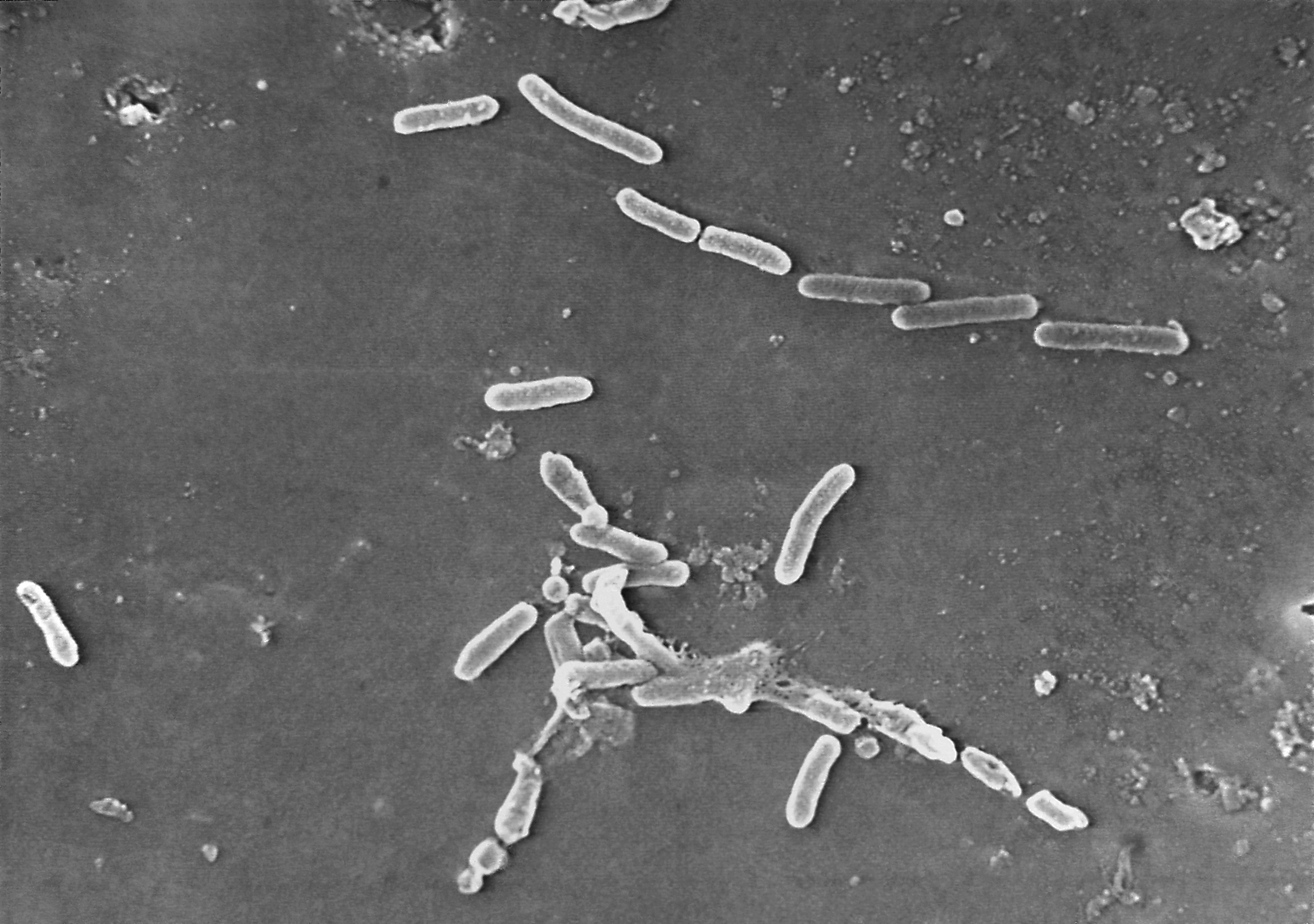
While there have been no reports of adverse reaction associated with using either of the products, the FDA says testing on a random sampling of the brands showed they were contaminated with microbes and were not sterile. The agency has been conducting routine testing of eyedrops amid a recent spate of deaths and vision loss from illnesses linked to eyedrops tainted with a drug-resistant bacteria.
At least 81 people have been infected with the bacteria, including four who died and 14 who lost vision, the Centers for Disease Control and Prevention said in May. The CDC also said four people have undergone surgery to remove an eyeball due to the infections.
After the recall, U.S. health inspectors visited the plant in India that made the eyedrops and uncovered problems with how the drops were made and tested, including inadequate sterility measures.
Cases have been reported from 18 states — California, Colorado, Connecticut, Delaware, Florida, Illinois, North Carolina, New Jersey, New Mexico, Nevada, New York, Ohio, Pennsylvania, South Dakota, Texas, Utah, Washington and Wisconsin.